Leading Pharmaceutical Firm Offering Oncology Products with Ethical Standards
Dedicated Oncology Facility
Admac Lifesciences is a dedicated oncology facility of Admac Group of Companies. Tablets, capsules, liquid and lyophilised injections are manufactured with precision under one roof. Admac Lifescences is the best oncology facility for the cancer drugs in India.
Certified Company
Admac Lifesciences is a WHO GMP certified facility. We are an ISO 9001:2000 certified cancer treatment drug making company.
R&D
We are endowed with a world class R&D, equipped with state of the art infrastructure, manufacturing units, chemical laboratories equipments, latest and expertised management for the development of oncology drugs for cancer treatment.
Passion
Generated with passion to serve health and quality solutions Admac Lifesciences, a part of Admac Group of Companies is a specialty pharmaceutical company dedicated to the oncology and cancer treatment drugs, engaged in the development, manufacturing and commercialization of pharmaceutical products at affordable prices.
Maximize Potential
We, at Admac Lifesciences aim to facilitate hospitals and other healthcare organizations to maximize their potential with our innovative quality healthcare and cancer treatment products.
Customised Solutions
We provide a wide array of customized solutions right from conceptualization, to planning , designing, to project management of hospitals and drugs used for cancer treatment with optimal operationalisation and clientele satisfaction.

Oncology
PRODUCTS & PROGRAMS
Our comprehensive oncology product basket comprises of fifty products. All our oncology products catering to cancer treatment are produced with strict adherence to ethical standards and global norms of W.H.O. GMP guidelines.
To extend physical and mental support to people grappling with the challenges of cancer, we have initiated several cancer care programs. The programs are intended to keep cancer patients and their families motivated during the course of their treatment by the anti cancer medicines. The concept is to convince them that in this strive against cancer, they are not alone.
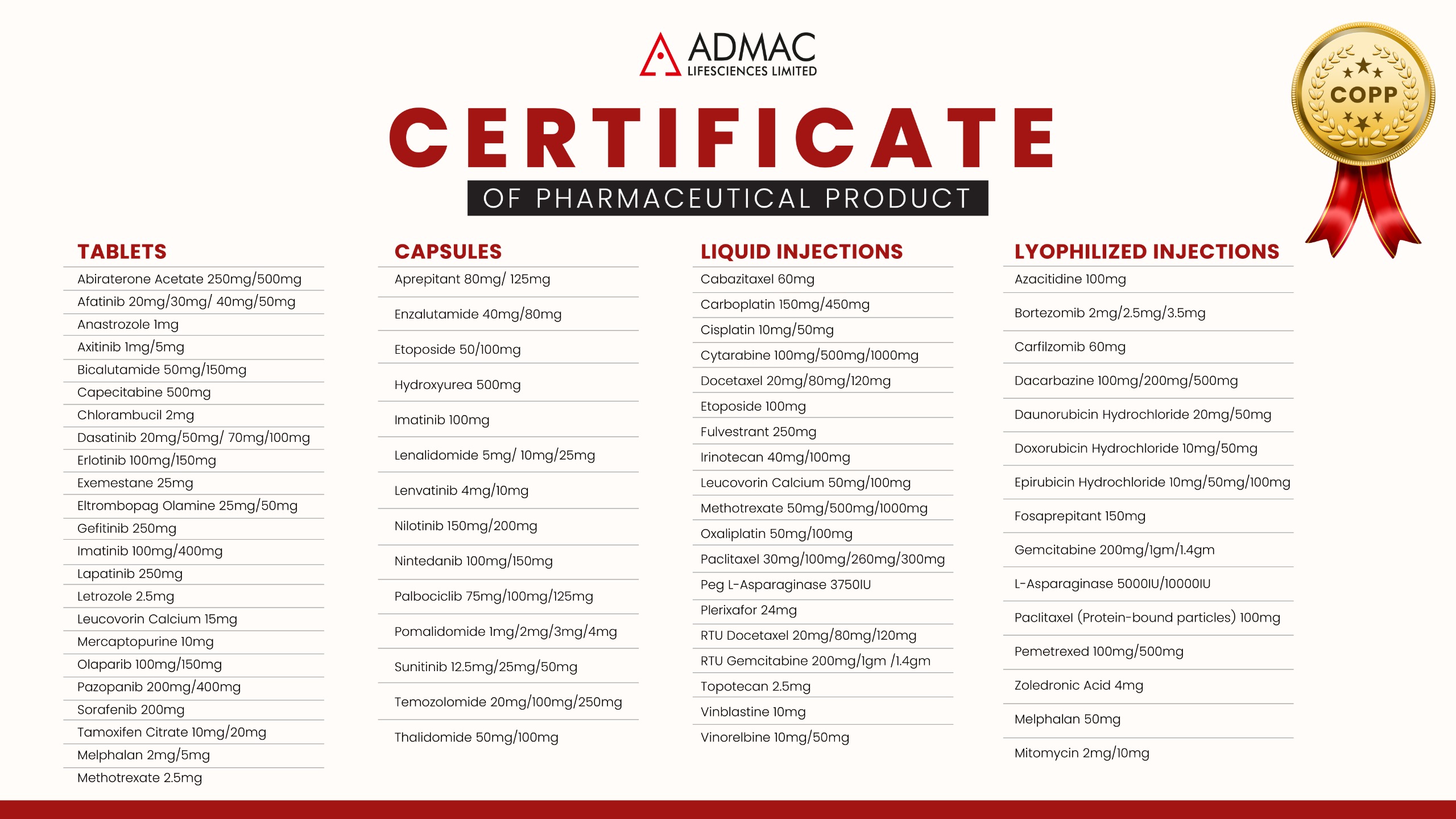
COPP
CERTIFICATES OF PHARMACEUTICAL PRODUCT
The COPP is the legal document that declares a certain manufacturing company is legally allowed to sell their pharmaceutical or an oncology product in the country they are producing. When registering a pharmaceutical product overseas, the government body in charge of approving the application will usually require a COPP to ensure that the product or chemotherapy medicines are being sold as a commercial finished product in the country that is producing it.
Admac Lifesciences has all its products and oncology medicines complying with the COPP standards- which includes lyophilised injections, liquid injections, tablets and capsules.

CERTIFICATIONS
ADMAC has state of the art manufacturing facilities for the development of anticancer medicine. All manufacturing facilities are designed as per GMP guidelines.
Being one of the fastest growing pharmaceutical companies for the cancer vaccine in India, we have always invested in building modern manufacturing facilities to increase our production capacities to meet the growing demand of our products.
Our anti cancer medicine manufacturing facilities are approved by WHO-GMP as well as the various regulatory bodies of the respective countries we operate in.
All facilities are equipped with advanced automation systems. HVAC system is designed with Variable Frequency Drive (VFD), Programmable Logic Controller (PLC) with automatic data recording monitors process control like air distribution, temperature, humidity, auto cleaning process, security by interlocking, auto loading, unloading and Cleaning-In-Place(CIP).
Our facilities have segregated de-humidified areas for manufacturing moisture-sensitive oncology products. Stainless steel panels are used for in-sterile facility core areas. The maintenance and servicing are done from the top of a false ceiling without entering the manufacturing areas.
Admac Lifesciences
EXPORT COUNTRIES
Admac’s state-of-the-art manufacturing facilities are designed as per USFDA guidelines. The facilities are constructed and designed with maximum consideration given to current Good Manufacturing Practices (cGMP). Admac Lifesciences foothold has also spread to international soil.
Our aim is to ensure better access of the affordable chemotherapy medicine. Thus, we have branched out to global markets so that we reach as many people as possible. Currently, we have a hold in 25+ countries across the globe with a wide range of products and anticancer medicines.

Our Leading Customer